Diane Pills Side Effects Explained: A Must-Read Guide
Table of Contents
Diane 35 is a widely used medication for various dermatological conditions and as a contraceptive. However, if you intend to use this medication, there are quite a few potential Diane 35 side effects as well as safety precautions that you should be aware of prior to using it.
Whether you are a new user or already taking DIANE 35, this will be your must-read guide that comprehensively reviews and analyzes its side effects, their occurrence, severity, and tips for management.
Therapeutic Indications
Diane 35 is primarily indicated for the treatment of certain androgen-dependent conditions in women, particularly:
- Severe Acne: It is prescribed for severe acne that hasn’t responded well to other anti-acne treatments including topical treatments and antibiotics.
- Polycystic Ovary Syndrome (PCOS): Diane 35 can help regulate menstrual cycles, reduce acne, and decrease excess hair growth (hirsutism) associated with PCOS.
- Hirsutism: Excessive hair growth in women caused by hormonal imbalances can be treated with Diane 35 thanks to its anti-androgenic effects.
-
Diane 35® Tablets
Cyproterone 2mg + Ethinyl Estradiol 0.035mg
Brand name: Alisma, Ancea, Axira, Bellgyn, Bellune, Chloe, Claudia, Diclin, Feminac
Size: 21 Tablets
USD $27.00 Add to cart -
Novelon® Tablets
Ethinyl Estradiol 0.03mg + Desogestrel 0.15mg
Size: 1 Stip of 21 Tablets
Brand name: Apri, Caziant, Cesia, Cyclessa, Kariva, Reclipsen, Solia, Velivet, Desogen
USD $18.00 Add to cart -
Femilon® Tablets
Ethinyl Estradiol 0.02mg + Desogestrel 0.15mg
Size: 1 Strip of 21 Tablets
Brand name: Viorele, Azurette, Bekyree, Caziant, Cyred, Juleber, Kalliga, Mircette
USD $19.00 Add to cart
Possible Side Effects
Even though DIANE-35 is known to have a high success rate in addressing the above conditions, occasionally, it may cause some side effects of varying severity. Therefore, if your overall health declines or you encounter any adverse effects while using DIANE-35 make sure to consult your healthcare provider for guidance promptly.
Common Side Effects
- Nausea
- Abdominal pain
- Breast pain and tenderness
- Headache
- Weight gain
- Changes in mood, including depression
Less Common Side Effects
- Vomiting
- Fluid retention
- Migraine
- Diarrhoea
- Breast discharge
- Vaginal discharge
- Changes in sexual interest
- Breast enlargement
- Rash
- Hives (urticaria)
- Blood clots in veins
- Allergic reactions
- Intolerance to contact lenses

Overdose
In the event of an overdose, Diane 35 could lead to manifestations such as nausea, vomiting, and cessation of bleeding. While severe adverse reactions resulting from overdose are rare, prompt medical attention is necessary if overdose symptoms occur.
Currently, antidotes for Diane 35 overdose are not available, and management typically focuses on alleviating symptoms and supportive care.
Interactions with Other Medications
As with any medication, Diane 35 may interact with other drugs, potentially altering its efficacy or increasing the risk of adverse effects. Therefore, users of DIANE 35 need to inform their healthcare provider of all medications, including over-the-counter drugs and herbal supplements, that they are taking concurrently with Diane 35 to avoid these drug-drug interactions.
Certain medications given for the following conditions may interact with Diane 35, affecting its metabolism and blood levels.
- bacterial infections (macrolide antibiotics, e.g. erythromycin)
- epilepsy (e.g. primidone, phenytoin, barbiturates, carbamazepine, oxcarbazepine, topiramate, felbamate)
- tuberculosis (e.g. rifampicin)
- fungal infections (griseofulvin, azole antifungals, e.g. itraconazole, voriconazole, fluconazole)
- certain heart diseases, high blood pressure (calcium channel blockers, e.g. verapamil, diltiazem)
Conversely, Diane 35 may also influence the effectiveness of other medications, particularly those metabolized by the liver or affecting hormone levels. Healthcare providers can provide guidance on potential drug interactions and adjust treatment regimens accordingly to ensure optimal therapeutic outcomes.
Warnings and Precautions
While Diane 35 is generally well-tolerated by most users, certain precautions should be taken to ensure its safe and effective use. Individuals with specific medical conditions or risk factors that are listed below may require special consideration before initiating Diane 35 therapy.
Smoking: Individuals who smoke cigarettes while using Diane 35 may be at an increased risk of developing cardiovascular complications, including blood clots, stroke, or heart attack. Therefore, smoking cessation is strongly advised, especially for users over the age of 35.
Diabetes: Patients with diabetes should be closely monitored while using Diane 35, as the medication may affect glucose metabolism and insulin sensitivity. Regular blood sugar monitoring and adjustment of diabetes medications may be necessary to maintain optimal glycemic control.
Cardiovascular Disorders: Individuals with pre-existing heart conditions, such as heart valve disorders or certain rhythm abnormalities, should exercise caution when using Diane 35. Close monitoring of cardiac function and periodic evaluation by a cardiologist may be warranted to minimize the risk of adverse cardiovascular events.
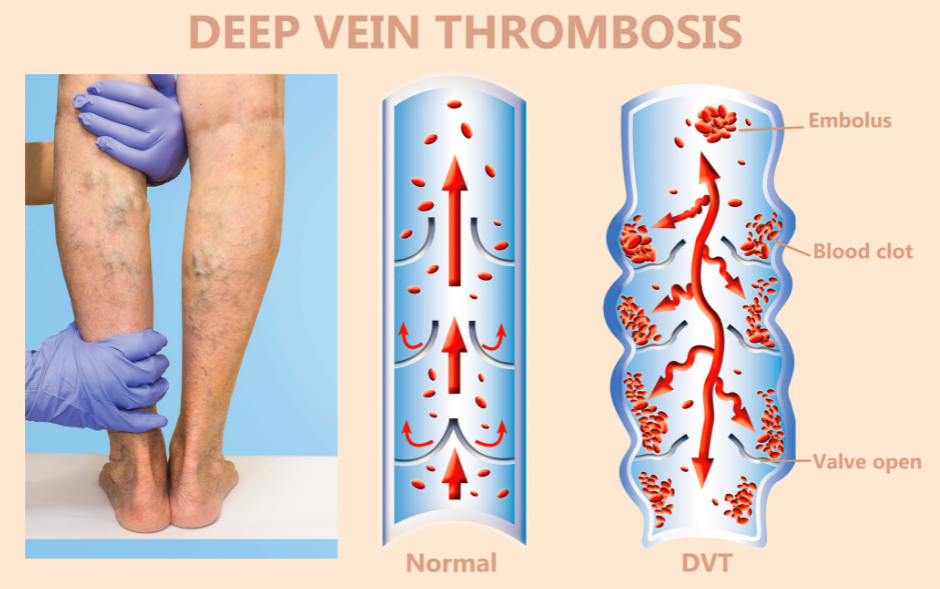
Venous Thromboembolism (VTE): Diane 35 has been associated with an increased risk of venous blood clots, including deep vein thrombosis (DVT) and pulmonary embolism (PE). Patients with a history of VTE or predisposing factors for thrombosis should be evaluated carefully before initiating Diane 35 therapy. Consideration should be given to alternative contraceptive methods in individuals at high risk of VTE.
Migraine: Women with a history of migraines may experience exacerbation or worsening of migraine symptoms while using Diane 35. Close monitoring of migraine frequency and intensity is recommended, and alternative treatment options should be considered if migraines become severe or frequent.
Obesity and Hypertension: Obesity and hypertension are risk factors for cardiovascular disease and may potentiate the adverse effects of Diane 35. Healthcare providers should assess the cardiovascular risk profile of obese or hypertensive individuals before prescribing Diane 35 and implement lifestyle modifications as appropriate.
Family History of Thrombosis or Cardiovascular Disease: Individuals with a family history of blood clots, heart attacks, or strokes at a young age may have an increased genetic predisposition to thrombosis or cardiovascular events.
Liver and Gallbladder Disorders: Patients with pre-existing liver or gallbladder disease may experience exacerbation of their condition while using Diane 35. Liver function tests should be performed before initiating Diane 35 therapy and periodically thereafter to monitor for hepatotoxicity or cholestasis. Diane 35 should be discontinued if significant liver dysfunction or gallbladder disease develops.
Other Medical Conditions: Individuals with certain medical conditions, such as systemic lupus erythematosus, Crohn’s disease, ulcerative colitis, or haemolytic uremic syndrome, may be at increased risk of adverse effects with Diane 35 use. Healthcare providers should exercise caution and consider alternative treatment options in such cases.
DIANE-35 and depression
Some women using hormonal contraceptives including DIANE-35 have reported depression or depressed mood. Depression can be serious and may sometimes lead to suicidal thoughts. If you experience mood changes and depressive symptoms, contact your doctor for further medical advice as soon as possible.

DIANE-35 and blood clots
When using DIANE-35, it’s important to be aware of the increased risk of blood clots, both in veins (venous thrombosis) and arteries. While this risk is slight, it’s still a crucial consideration. Venous thrombosis can lead to blockages in the veins of the legs, lungs (resulting in pulmonary embolism), or other organs, potentially causing severe complications.
Similarly, arterial clots can have serious consequences, such as heart attacks or strokes, depending on their location.
Several factors can further elevate the risk of blood clots for individuals taking DIANE-35. Age plays a significant role, with older individuals facing higher risks. Smoking also substantially increases the likelihood of blood clot formation, especially for those over 35.
Additionally, factors like obesity, high blood pressure, family history of heart issues, elevated fat levels, migraines, and pre-existing heart conditions can exacerbate the risk.
It’s crucial for those using DIANE-35 to maintain open communication with their healthcare providers, particularly if they need surgery or experience extended periods of immobility due to injury or illness.
In such cases, treatment adjustments may be necessary, including temporary discontinuation of the contraceptive. Furthermore, quitting smoking is strongly advised, particularly for older users, to mitigate the heightened risk of blood clots associated with both age and smoking.
DIANE-35 and Cancer
While breast cancer rates are slightly elevated in women using combined pills, the direct causative link to the treatment remains uncertain. It’s plausible that heightened vigilance and more frequent medical check-ups among pill users contribute to this observation.
Notably, the risk of breast tumors diminishes gradually post-discontinuation of combined hormonal contraceptives. Therefore, regular breast self-examinations are crucial, with any detected lumps warranting immediate medical attention.

Additionally, rare occurrences of benign liver tumors and even fewer cases of malignant liver tumors have been documented in contraceptive pill users, occasionally leading to severe internal bleeding. Hence, any instance of unusually severe abdominal pain should prompt consultation with a healthcare provider.
Furthermore, persistent Human papillomavirus (HPV) infection stands as the primary risk factor for cervical cancer. Although some studies suggest a potential elevation in cervical cancer risk with long-term pill use, the precise contribution of factors like sexual behavior and HPV infection to this increased risk remains unclear. Nonetheless, these tumors can pose life-threatening risks, underscoring the importance of prompt medical evaluation and intervention.
Bleeding Between Periods
During the initial months of Diane-35 use, you may experience irregular vaginal bleeding, such as spotting or breakthrough bleeding, necessitating the use of sanitary protection. It’s important to continue taking your tablets as prescribed.
Typically, irregular vaginal bleeding subsides once your body adjusts to Diane-35, usually within about three tablet-taking cycles. However, if the bleeding persists, becomes heavy, or recurs, it’s advisable to inform your doctor promptly.
If No Bleeding Occurs: If you’ve taken all the tablets correctly for one cycle, haven’t experienced vomiting or severe diarrhoea, and haven’t taken any other medications, the likelihood of pregnancy is low. In such cases, continue taking Diane-35 as usual. However, if you’ve taken the tablets incorrectly or if expected bleeding doesn’t occur twice in a row despite correct tablet intake, pregnancy is a possibility.
Contact your doctor immediately for further assessment. Until pregnancy is ruled out, refrain from starting the next pack and use non-hormonal contraceptive measures as a precautionary measure.
Pregnancy and Breastfeeding
Diane-35 should not be taken if you are pregnant or suspect you may be pregnant. If pregnancy occurs while using Diane-35, discontinue the medication immediately and consult your doctor. If you’re planning to conceive, you can stop taking Diane-35 at any time. However, it’s important to note that Diane-35 must not be used during breastfeeding due to potential risks to the infant.
Conclusion
In conclusion, Diane 35 is a valuable medication for the management of various dermatological conditions and contraception in women. However, like all medications, it is associated with potential side effects and risks that require careful consideration by healthcare providers and patients alike.
By adhering to recommended precautions, monitoring patients closely, and promptly addressing any adverse effects that may arise, healthcare providers can ensure the safe and effective use of Diane 35 in eligible individuals.
References
- Poli, G., Cordiali-Fei, P., & Pichini, S. (2020). Acne and contraception: A comprehensive review. Dermatologic Therapy, 33(6), e13887. [DOI: 10.1111/dth.13887]
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. (2004). Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertility and Sterility, 81(1), 19-25. [DOI: 10.1016/j.fertnstert.2003.10.004]
- Mortada, R., & Williams, T. (2015). Metformin use in polycystic ovary syndrome. Annals of Translational Medicine, 3(19), 287. [DOI: 10.3978/j.issn.2305-5839.2015.10.32]
- Yildiz, B. O., Bozdag, G., & Yapici, Z. (2012). The efficacy of metformin and clomiphene citrate combination compared with clomiphene citrate alone for ovulation induction in infertile patients with PCOS. Gynecological Endocrinology, 28(7), 605-608. [DOI: 10.3109/09513590.2011.651134]
- Mastorakos, G., & Lambrinoudaki, I. (2006). Management of endocrine disease: Menopausal transition and the risk of cardiovascular disease. European Journal of Endocrinology, 154(2), 173-182. [DOI: 10.1530/eje.1.02118]
- Simon, J. A., & Shangold, G. A. (2000). Risks and benefits of oral contraceptive use in women older than 35 years of age. Obstetrics and Gynecology Clinics of North America, 27(4), 741-754. [DOI: 10.1016/s0889-8545(05)70166-7]
- Lidegaard, Ø., & Nielsen, L. H. (2008). Increased risk of venous thromboembolism during the use of hormonal contraceptives: A systematic review and meta-analysis. Human Reproduction Update, 14(2), 119-138. [DOI: 10.1093/humupd/dmm035]
- Lidegaard, Ø., Løkkegaard, E., Svendsen, A. L., & Agger, C. (2009). Hormonal contraception and risk of venous thromboembolism: National follow-up study. BMJ, 339, b2890. [DOI: 10.1136/bmj.b2890]
- Beral, V., Bull, D., Reeves, G., & Million Women Study Collaborators. (2007). Endometrial cancer and hormone-replacement therapy in the Million Women Study. The Lancet, 369(9573), 1703-1710. [DOI: 10.1016/s0140-6736(07)60534-0]
- Hannaford, P. C., Selvaraj, S., Elliott, A. M., & Angus, V. (2010). Itraconazole interaction with low dose combined oral contraceptive pills. British Journal of Clinical Pharmacology, 69(2), 176-178. [DOI: 10.1111/j.1365-2125.2009.03585.x]
Post by:
Dr.Marcella Jiovanni
Health and Beauty Expert
“Marcella Jiovanni actively promotes the importance of maintaining healthy skin, she envisions the future of dermatology as moving away from pure medical, pharmacological dermatology and flowing more toward a holistic approach to wellness and skincare.”