Description
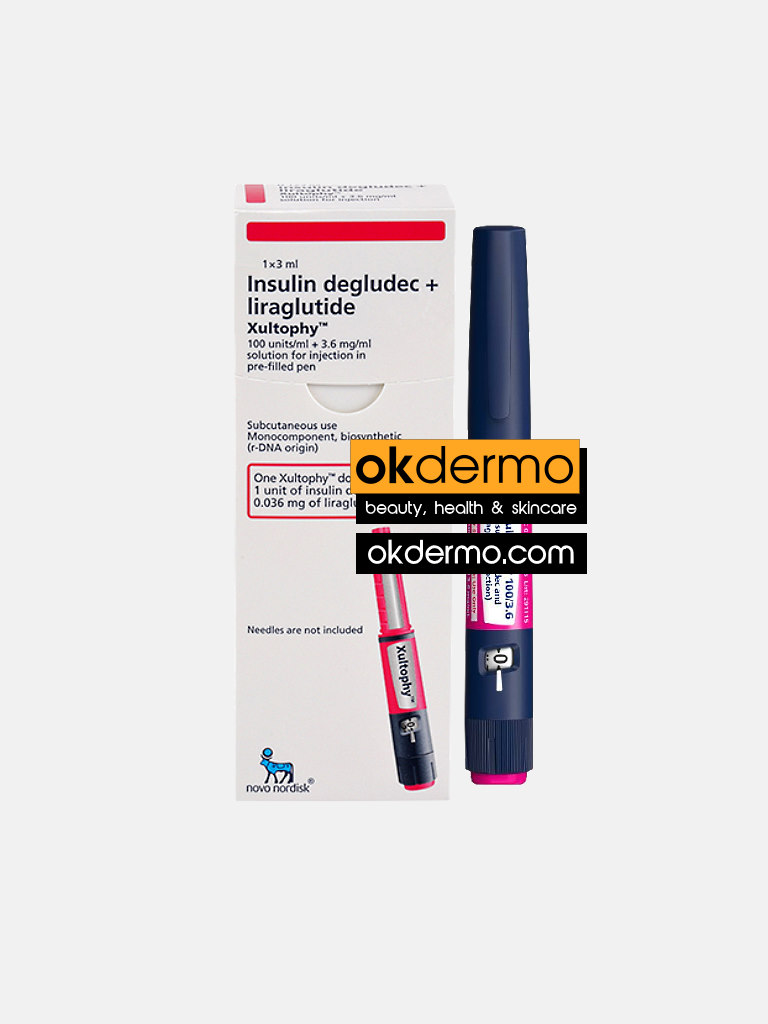
What is Xultophy® Pre-Filled Pen Injection?
Xultophy Injection is a combination medicine used in the treatment of type 2 diabetes mellitus. It is usually given when your blood sugar levels have not been well controlled by using other medications. This medicine works best when used together with diet and exercise.
Xultophy Injection should be used in the dose and duration as prescribed by your doctor. Your doctor or nurse will teach you the correct way of injecting it under the skin. You should use this medicine regularly as per the dose advised to get the most benefit. If you stop taking it your blood sugar levels may rise very high and put you at risk of serious complications. It is only part of a treatment program that should also include a healthy diet, regular exercise, and weight reduction as advised by your doctor. Follow the instructions given by your doctor carefully.
You may experience nasal congestion, sore throat, headache, respiratory tract infection, nausea, increased lipase in the blood, and diarrhea. Additionally, you may notice some injection site reactions like pain, swelling, or redness. Consult your doctor if these do not resolve or persist for a longer duration.
Before taking this medicine, inform your doctor if you have any kidney, liver, or heart problems. Pregnant or breastfeeding women should also consult their doctor before taking it. Your doctor will check your kidney function tests before starting treatment with it. Avoid excessive alcohol intake while taking it as this may increase the risk of developing some side effects.
Warnings and Precautions
Risk of Thyroid C-cell Tumors: If serum calcitonin is measured and found to be elevated or thyroid nodules are noted on physical examination or neck imaging, the patient should be further evaluated.
Pancreatitis: Acute pancreatitis, including fatal and non-fatal hemorrhagic or necrotizing pancreatitis, has been observed in patients treated with liraglutide postmarketing. Observe patients carefully for signs and symptoms of pancreatitis (persistent severe abdominal pain, sometimes radiating to the back with or without vomiting). If pancreatitis is suspected, discontinue Xultophy® 100/3.6 promptly and if pancreatitis is confirmed, do not restart. Liraglutide, one of the components of Xultophy® 100/3.6, has been studied in a limited number of patients with a history of pancreatitis. It is unknown if patients with a history of pancreatitis are at a higher risk for the development of pancreatitis on liraglutide. Never Share a Xultophy® 100/3.6 Pen Between Patients, even if the needle is changed. Sharing of the pen poses a risk for the transmission of blood-borne pathogens.
Hyperglycemia or Hypoglycemia with Changes in Insulin Regimen: Changes in an insulin regimen (e.g., insulin strength, manufacturer, type, or injection site or method of administration) may affect glycemic control and predispose to hypoglycemia or hyperglycemia. Repeated insulin injections into areas of lipodystrophy or localized cutaneous amyloidosis have been reported to result in hyperglycemia, and a sudden change in the injection site (to an unaffected area) has been reported to result in hypoglycemia. Make any changes to a patient’s insulin regimen under close medical supervision with increased frequency of blood glucose monitoring. Advise patients who have repeatedly injected into areas of lipodystrophy or localized cutaneous amyloidosis to change the injection site to unaffected areas and closely monitor for hypoglycemia. Adjustments in concomitant anti-diabetic treatment may be needed.
Overdose Due to Medication Errors: Instruct patients to check the label before each injection since accidental mix-ups with insulin-containing products can occur. Do not administer more than 50 units of Xultophy® 100/3.6 daily. Do not exceed the 1.8 mg maximum recommended dose of liraglutide or use with other GLP-1 RAs.
Hypoglycemia: Hypoglycemia is the most common adverse reaction of insulin-containing products, including Xultophy® 100/3.6, and may be life-threatening. Increase monitoring with changes to dose, co-administered glucose-lowering medications, meal pattern, physical activity; and in patients with hypoglycemia unawareness or renal or hepatic impairment.
Acute Kidney Injury: Acute renal failure and worsening of chronic renal failure, which may sometimes require hemodialysis, have been reported postmarketing for liraglutide, usually in association with nausea, vomiting, diarrhea, or dehydration. Advise patients of the potential risk of dehydration due to gastrointestinal adverse reactions and take precautions to avoid fluid depletion.
Hypersensitivity and Allergic Reactions: Severe, life-threatening, generalized allergy, including anaphylaxis, angioedema, bronchospasm, hypotension, and shock can occur. If a hypersensitivity reaction occurs, discontinue and treat per standard of care. Anaphylaxis and angioedema have been reported with other GLP-1 RAs. Use caution in a patient with a history of anaphylaxis or angioedema with other GLP-1 RAs because it is unknown whether such patients will be predisposed to these reactions with Xultophy® 100/3.6.
Acute Gallbladder Disease: In a cardiovascular outcomes trial (LEADER trial) 3.1% of patients treated with liraglutide, one of the components of Xultophy® 100/3.6, versus 1.9% of placebo-treated patients reported an acute event of gallbladder diseases, such as cholelithiasis or cholecystitis. The majority of events required hospitalization or cholecystectomy. If cholelithiasis is suspected, gallbladder studies and appropriate clinical follow-up are indicated.
Hypokalemia: All insulin-containing products, including Xultophy® 100/3.6 can lead to life-threatening hypokalemia, which may then cause respiratory paralysis, ventricular arrhythmia, and death. Monitor potassium levels in patients at risk for hypokalemia and treat if indicated.
Fluid Retention and Congestive Heart Failure: Patients using insulin-containing products, including Xultophy® 100/3.6, with thiazolidinediones (TZDs) should be observed for signs and symptoms of heart failure. If heart failure develops, dosage reduction or discontinuation of the TZD must be considered.
Adverse Reactions and Drug Interactions
The most common adverse reactions, reported in ≥5% of patients treated with Xultophy® 100/3.6 are nasopharyngitis, headache, nausea, diarrhea, increased lipase, and upper respiratory tract infection.
Certain drugs may affect glucose metabolism, requiring dose adjustment and close monitoring of blood glucose. The signs and symptoms of hypoglycemia may be reduced or absent in patients taking anti-adrenergic drugs (e.g., beta-blockers, clonidine, guanethidine, and reserpine).
Liraglutide-containing products, including Xultophy® 100/3.6, cause a delay of gastric emptying and thereby have the potential to impact the absorption of concomitantly administered oral medications. Caution should be exercised when oral medications are concomitantly administered with liraglutide-containing products.