Description
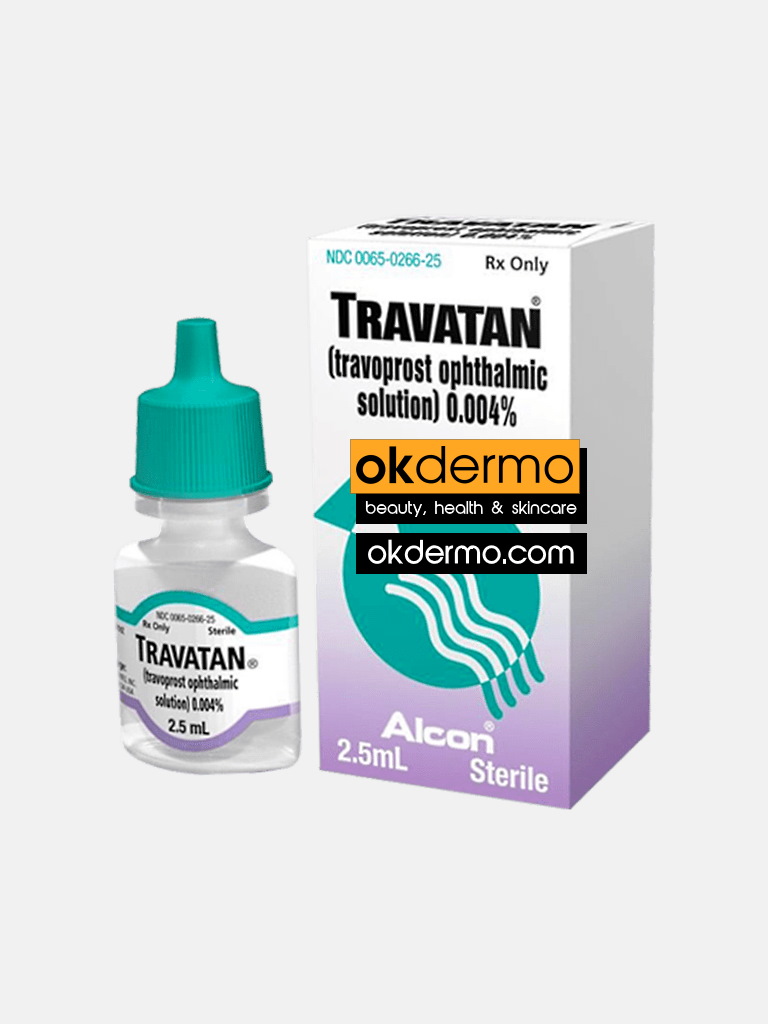
What is Travoprost Ophthalmic Solution 0.004% (4mcg/ml)
Travatan Eye Drop is used for the treatment of glaucoma or ocular hypertension. Travoprost Ophthalmic Solution 0.004% is the main active component of the drops and it works to relieve the high pressure inside the eye caused by the excessive outflow of fluid from the eye which then relieves the pressure. Travatan Eye Drop works by decreasing the pressure in the eyeball. Travatan Eye Drops do this by increasing the drainage of aqueous humor which is the clear watery fluid filling the space in front of an eyeball. This reduces pressure on the eye relieving the symptoms of pain.
It is a synthetic prostaglandin analog (or more specifically, an analog of prostaglandin F2α) that works by increasing the outflow of aqueous fluid from the eyes. Like other analogs of prostaglandin F2α such as tafluprost and latanoprost, travoprost is an ester prodrug of the free acid, which acts as an agonist at the prostaglandin F receptor, increasing outflow of aqueous fluid from the eye and thus lowering intraocular pressure.
Travatan eyedrop is a treatment for high intraocular pressure, particularly glaucoma. Travatan solution contains travoprost, which is a prostaglandin analog. Prostaglandin-like molecules are the safest and most efficient treatment for glaucoma. Ophthalmologists and medical doctors prescribe Travatan as a first-line treatment for open-angle glaucoma, particularly in patients whose disease could not be controlled with other agents.
Travoprost activity occurs after two hours of application and provides a lasting pressure-lowering diurnal effect. Prostaglandins are a large family of lipid compounds with many subtypes that have multiple hormone-like activities in the body. Prostaglandin analogs lower the intraocular pressure by promoting the aqueous humor egress through both its natural drainage pathways.
How to use Travatan Travoprost Drop 0.004%
Your eye doctor will tell you how much of this medicine to use and how often. Do not use more medicine or use it more often than your doctor tells you to. Your doctor may order 2 or more eye drops to be used together. You should wait at least 5 minutes before putting another eye drop in the same eye. Remove contact lenses before using this medicine. Wait at least 15 minutes after using this medicine before putting the contact lenses back in.
- The bottle is only partially full to provide proper drop control.
- First, wash your hands. Tilt your head back and, pressing your finger gently on the skin just beneath the lower eyelid, pull the lower eyelid away from the eye to make a space. Drop the medicine into this space. Let go of the eyelid and gently close the eyes.
- Do not blink. Keep the eyes closed for 1 to 2 minutes to allow the medicine to cover the eye.
- Remove any excess solution around the eye with clean tissue, being careful not to touch the eye.
- Immediately after using the eye drops, wash your hands to remove any medicine that may be on them.
- To keep the medicine as germ-free as possible, do not touch the applicator tip to any surface (including the eye). Also, keep the container tightly closed.
Travatan Therapeutic Indications
Travatan is used in the treatment of open-angle glaucoma and high intraocular pressure. Several medical agents can treat glaucoma. Either by increasing the egress of aqueous humor or by reducing its production.
Travoprost is a prostaglandin analog that acts positively on aqueous humor drainage. Enhancing the outflow of intraocular fluids restores equilibrium between production and egress, resulting in decreased tension inside the eyes.
Glaucoma is a disease where the optic nerve gets progressively damaged. It is the leading cause of visual acuity, irreversible disturbance, and the second leading cause of blindness.
Patients with open-angle glaucoma are often asymptomatic until the visual loss becomes prominent unless the disease is depicted with a routine ophthalmic check-up.
The main modifiable risk factor is intraocular pressure. Control over the intraocular pressure permits easier management of the disease and helps prevent further disease progression.
Despite glaucoma being uncurable and vision loss is irrecuperable. Several studies showed that medical treatment significantly impacts the progression into blindness.
Even a slight reduction in intraocular pressure results in better outcomes.
A study showed that one mmHg reduction in intraocular pressure is associated with a more than 10% chance of preventing visual loss from baseline.
Therefore, any therapeutic agent that can reduce intraocular pressure is of significant value in glaucoma treatment.
The newer agents in the glaucoma setting are prostaglandin analogs. They bind to prostaglandin receptors located in both the ciliary muscle and uveoscleral pathway. Travoprost first entered the market in 2001 and was approved by the FDA the same year as a safe medication for open-angle glaucoma.
Several studies showed that topical treatment with Travatan results in a 10 to 30% reduction in intraocular pressure. A significant effect that nearly totally impedes the damage to the optic neurons and blocks disease progression.
Travoprost intraocular pressure-lowering effect has a dual mechanism. It acts on both aqueous humor production and egress to restore the pressure balance inside the eye.
Travoprost promotes aqueous humor egress through the trabecular meshwork and the uveoscleral pathway. It does so by remodeling the structure of the trabecular meshwork. So it becomes more permeable to the aqueous humor.
Travoprost also acts on the ciliary body to decrease aqueous humor secretion. This mechanism is more significant with prolonged use.
Travoprost is relatively safe compared with the rest of glaucoma medications. Side effects include itchiness, redness, stinging sensation, and dry eyes.
Side effects of Travatan Travoprost Drop 0.004%
Along with its needed effects, a medicine may cause some unwanted effects. Although not all of these side effects may occur, if they do occur they may need medical attention. Check with your doctor immediately if any of the following side effects occur: eye pain, itching eye, redness of the eye, redness, swelling, or itching of the eyelid, blurred or decreased vision, burning, dry, or itching eyes, eye color changes, redness, pain, or swelling of the eye, eyelid, or inner lining of the eyelid.
Some side effects may occur that usually do not need medical attention. These side effects may go away during treatment as your body adjusts to the medicine. Also, your health care professional may be able to tell you about ways to prevent or reduce some of these side effects.