Description
What is Tranexamic Acid 500mg Tablets
What is Tranexamic Acid? A synthetic derivative of the amino acid lysine, tranexamic acid has been used by physicians to address skin discoloration via the topical application and orally. This ingredient has been shown to diminish the appearance of discoloration and improve stubborn brown patches.
Tranexamic Acid is widely used in dermatology to address skin discoloration/melasma, tranexamic acid is a powerful ingredient recognized for its ability to brighten skin complexion and improve the appearance of discoloration.
Tranexamic acid is a procoagulant agent that is approved by the US Food and Drug Administration for the treatment of menorrhagia and to prevent hemorrhage in patients with hemophilia undergoing tooth extractions. Heavy menstrual bleeding, previously known as menorrhagia, is a menstrual period with excessively heavy flow. It is a type of abnormal uterine bleeding (AUB). Abnormal uterine bleeding can be caused by structural abnormalities in the reproductive tract, anovulation, bleeding disorders, hormone issues (such as hypothyroidism), or cancer of the reproductive tract. The initial evaluation aims at figuring out pregnancy status, menopausal status, and the source of bleeding. Treatment depends on the cause, severity, and interference with quality of life.
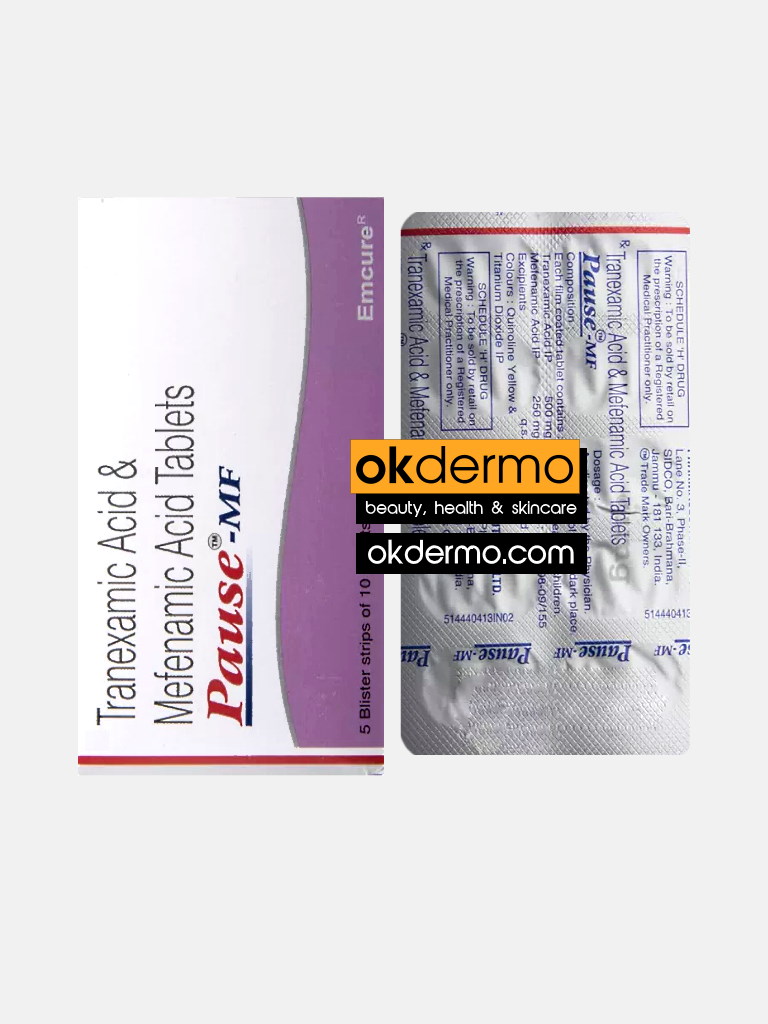
Pause-MF® Tablets is a combination medicine used to treat heavy menstrual bleeding and menstrual pain. It prevents the breakdown of blood clots to control excessive bleeding during periods. It also blocks the production of certain chemical messengers that cause pain and inflammation. Pause-MF® Tablet is a combination of two medicines: Tranexamic Acid and Mefenamic Acid. Tranexamic Acid is an anti-fibrinolytic. It works by preventing the breakdown of blood clots to control excessive bleeding during periods. Mefenamic Acid is a non-steroidal anti-inflammatory drug (NSAID) that blocks the production of certain chemical messengers (prostaglandins) that cause pain and inflammation (redness and swelling).
Through its inhibitory effects on the plasminogen activation pathway, tranexamic acid also mitigates the UV radiation-induced pigmentation response. Systemic tranexamic acid has consistently been reported as an effective treatment of melasma, though its broad use may be limited by the risk for thromboembolism. Limited studies have investigated the efficacy of topical tranexamic acid, with or without the use of adjunctive therapies to increase uptake. Melasma is a localized, chronic acquired hypomelanosis of the skin. Despite several treatments available, melasma can often be refractory to treatment. Also, recurrences are common. Tranexamic acid is a new addition in the treatment of melasma and is effective in a dosage of 250 mg BD for at least 4 to 8 weeks. Tranexamic acid acts mainly via the plasminogen activator-plasmin system to prevent UV radiation-induced pigmentation in melasma.
Tranexamic acid is a synthetic lysine derivative that inhibits plasminogen activation by blocking lysine-binding sites on the plasminogen molecule. Although the US Food and Drug Administration–approved indications for tranexamic acid include treatment of patients with menorrhagia and reduction or prevention of hemorrhage in patients with hemophilia undergoing a tooth extraction, the potential efficacy of tranexamic acid in the treatment of melasma has been consistently reported since the 1980s.
Tranexamic acid exerts effects on pigmentation via its inhibitory effects on UV light-induced plasminogen activator and plasmin activity. UV radiation induces the synthesis of plasminogen activators by keratinocytes, which results in increased conversion of plasminogen to plasmin. Plasminogen activator induces tyrosinase activity, resulting in increased melanin synthesis. The presence of plasmin results in increased production of both arachidonic acid and fibroblast growth factors, which stimulate melanogenesis and neovascularization, respectively. By inhibiting plasminogen activation, tranexamic acid mitigates UV radiation-induced melanogenesis and neovascularization. In treated guinea pig skin, the application of topical tranexamic acid following UV radiation exposure inhibited the development of expected skin hyperpigmentation and also reduced tyrosinase activity.
The largest study on the use of oral tranexamic acid for the treatment of melasma was a retrospective chart review of 561 melasma patients treated with tranexamic acid at a single center in Singapore. More than 90% of patients received prior treatment of their melasma, including bleaching creams and energy-based treatment. Among patients who received oral tranexamic acid over a 4-month period, 90% of patients demonstrated improvement in their melasma severity. Side effects were experienced by 7% of patients; the most common side effects were abdominal bloating and pain (experienced by 2% of patients). Notably, 1 patient developed deep vein thrombosis during treatment and subsequently was found to have protein S deficiency.
Although the daily doses of tranexamic acid for the treatment of menorrhagia and perioperative hemophilia patients are 3900 mg and 30 to 40 mg/kg, respectively, effective daily doses reported for the treatment of melasma have ranged from the initial report of efficacy at 750 to 1500 mg to subsequent reports of improvement at daily doses of 500 mg.
Challenges to the use of tranexamic acid for melasma treatment in the United States include specifically the risks associated with using a systemic procoagulant medication for a cosmetic indication. Patients should be screened and counseled on the risks of developing deep vein thrombosis and pulmonary embolism prior to initiating treatment. Cost and accessibility also may limit the use of tranexamic acid in the United States.
Given the potential for serious adverse effects with the use of systemic tranexamic acid, there has been interesting in formulating and evaluating topical tranexamic acid for cosmetic indications. Topical tranexamic acid has been used alone and in conjunction with modalities to increase uptake, including intradermal injection, micro-needling, and fractionated CO2 laser. Although these reports show initial promise, the currently available data are limited by small sample sizes, short treatment durations, lack of dose comparisons, and lack of short-term or long-term follow-up data. In addition to addressing these knowledge gaps in our understanding of topical tranexamic acid as a treatment option for melasma, further studies on the minimum systemic dose may address the downside of cost and potential for complications that may limit the use of this medication in the US.
The potential uses for tranexamic acid extend to the treatment of postinflammatory hyperpigmentation and rosacea. Melanocytes cultured in media conditioned by fractionated CO2 laser-treated keratinocytes were found to have decreased tyrosinase activity and reduced melanin content when treated with tranexamic acid, suggesting the potential role for tranexamic acid to be used postprocedural to reduce the risk for postinflammatory hyperpigmentation in prone skin types. Oral and topical tranexamic acid also has been reported to improve the appearance of erythematotelangiectatic rosacea, potentially relating to the inhibitory effects of tranexamic acid on neovascularization. Although larger-scale controlled studies are required for further investigation of tranexamic acid for these indications, it has shown early promise as an adjunctive treatment for several dermatologic disorders, including melasma, and warrants further characterization as a potential therapeutic option.
What is Tranexamic Acid 250mg Tablets Nexna TX®?
Nexna TX® consists of Tranexamic Acid I.P. 250mg and is generally used for the treatment of bleeding. Nexna TX® tablets is an anti-fibrinolytic that prevents the breakdown of blood clots, this controls excessive bleeding during or after surgery and also in other conditions. Nexna TX® is used to treat menorrhagia which is known as heavy menstrual bleeding. Nexna TX® is also used on a short term basis to treat patients affected by hemophilia.
Nexna TX is used to prevent or reduce bleeding for a short period of time in conditions like heavy periods, dysfunctional uterine bleeding, nosebleed, tooth removal, after prostate surgery, or after bladder surgery. Nexna TX works by increasing the clotting action of the blood to slow or stop active bleeding.
The most common side effects may be injection site reactions such as tiredness, nasal congestion, or pain in muscle, bone, or joint. Inform your doctor if you have undergone any cardiac surgery or you are suffering from any kidney disease.
Nexna TX® is an effective, trusted and safe systemic therapy. Tranexamic Acid in Nexna TX® tablets is generally used for the treatment of bleeding but new research/studies showed it effectiveness in skin hyperpigmentation/melasma treatment.
Tranexamic Acid is one of the modalities that can actually prevent the activation of melanocytes by sunlight, hormonal influence, and injured keratinocyte. Nexna TX® can not only reduce the pigmentation of melasma but also reduce the likelihood of recurrence.
Indications:
- Melasma/Chloasma
- UV induced hyperpigmentation
- Post-inflammatory hyperpigmentation
- Dysfunctional uterine bleeding, nosebleed, tooth removal
- Heavy menstrual bleeding
What is Tranexamic Acid 250mg Tablets Procdina TX®
Tranexamic acid is used to treat heavy menstrual bleeding in women. This medicine may be used by teenage females, but is not intended for use before the start of menstruation. Tranexamic acid is an antifibrinolytic agent. It works by blocking the breakdown of blood clots, which prevents bleeding.
Tranexamic Acid is used to treat bleeding. It helps to prevent or reduce bleeding in conditions like tooth removal, heavy periods, dysfunctional uterine bleeding, nosebleed, and in any oral, prostate, or bladder surgery.
Melano-TX OD Tablet SR is an anti-fibrinolytic. It works by preventing the breakdown of clots which leads to the stoppage of bleeding. This medicine must be taken in the dose and duration as advised by the doctor. You should not take it if you have any known allergy to this medicine.
The most common side effects include tiredness, nasal congestion, and pain in muscles, bones, or joints. Inform your doctor if you have undergone any cardiac surgery or you are suffering from any kidney disease. This medicine is safe to use in pregnant or breastfeeding mothers. It is also safe to use in patients suffering from liver diseases. It is advised not to consume alcohol after taking this medicine as there can be excessive drowsiness.
Oral tranexamic acid lightens refractory melasma
Melasma is a common acquired hyperpigmentary disorder, particularly among Asians and Hispanics, but its exact pathomechanism is poorly understood. Tranexamic acid has been found to lighten melasma by interfering with the interaction of melanocytes and keratinocytes by inhibiting the plasminogen/plasmin system. The aim was to evaluate the therapeutic effects of oral tranexamic acid in the treatment of melasma refractory to topical skin-lightening agents.
The retrospective study analyses patients with melasma recruited from a tertiary dermatological center in Singapore between 1 August 2009 and 31 March 2011. The patients chosen had refractory melasma treated with oral tranexamic acid 250 mg twice daily in addition to pre-existing combination topical therapy. Objective assessment using the physician’s global assessment and melasma area and severity index (MASI) scores were performed based on a post-hoc analysis of photographic records by three independent physicians. A paired t-test was used to evaluate the changes in the MASI scores pre-therapy and post-treatment. Statistical significance was defined as P < 0.05.
Altogether 25 patients were treated with tranexamic acid for a mean period of 3.7 ± 0.33 months, in addition to combination topical therapy. Their mean age was 47.2 ± 1.61 years. The mean MASI scores after tranexamic acid treatment (2.7 ± 1.6) were significantly lower (P < 0.01) than those prior to treatment (8.8 ± 4.2). The mean improvement in scores was 69%. The follow-up period was up to 6 months. Low-dose oral tranexamic acid can serve as a safe and useful adjunct in the treatment of refractory melasma.
Benefits of Tranexamic Acid
When shopping for products that target skin discoloration, it is important to look for well-studied ingredients backed by clinical results. Tranexamic acid is one such ingredient. Used and recommended by dermatologists, it works by addressing the appearance of discoloration at its onset. Studies have also demonstrated its ability to address hard-to-treat forms of discoloration including post-acne marks and stubborn brown patches. Tranexamic acid can be combined with other discoloration-fighting ingredients that work synergistically to create a multi-action approach targeting discoloration.
To understand how tranexamic acid helps brighten skin, it is important to understand why skin discoloration occurs in the first place. Everyday elements – including UV exposure, prescription drugs, and some professional treatments – can trigger the skin discoloration process by activating inflammatory mediators within the skin. This results in a chain reaction that causes the skin to produce melanin, the naturally-occurring pigment that gives skin its color. While the purpose of melanin is protective, when produced in excess, it can form deposits in the skin that manifest as dark patches, isolated spots, or an overall darker facial appearance. Tranexamic acid is a potent ingredient that helps reduce the appearance of discoloration and improves the look of stubborn brown spots, resulting in an overall brighter appearance.
Tranexamic acid is a synthetic amino acid derived from lysine. Topically tranexamic acid works by interrupting at least two pathways in skin that, left unchecked, can lead to discolorations, including larger patches known as melasma. Tranexamic acid also seems to work within the skin’s surface layers to make skin colorless susceptible to UV light exposure (but of course, you still need sunscreen) and may also reduce redness. Recent, double-blind and comparative research has shown that topical tranexamic acid in amounts between 2-5% rivals results of over-the-counter amounts of hydroquinone, long considered the gold standard for fading skin discolorations. If you’re using a hydroquinone product for lightening, you can add a product with tranexamic acid and potentially see even better results.
Comparative studies have shown tranexamic acid has greater tolerability than hydroquinone, although the latter is generally well tolerated when used as directed. Research has shown that tranexamic acid is safe when applied to skin every day for several months. Topical concentrations between 2-5% typically shows results after two to three months of once- or twice-daily usage. Because tranexamic acid is a water-soluble ingredient, it needs to be used in skincare products with oil-soluble ingredients designed to improve its penetration into the skin.
It’s worth mentioning that tranexamic acid is sometimes prescribed for oral use to women experiencing heavy periods or to those with various clotting disorders. It’s also used orally in low doses to manage signs of melasma. Research has revealed that how tranexamic acid works in the body to prevent excessive bleeding is very different from how it works topically as a cosmetic ingredient meant to visibly fade discolorations. Tranexamic acid skin whitening reviews may check on OKDERMO.com.
References:
Topical tranexamic acid as a promising treatment for melasma
Tranexamic Acid in Melasma: A Review