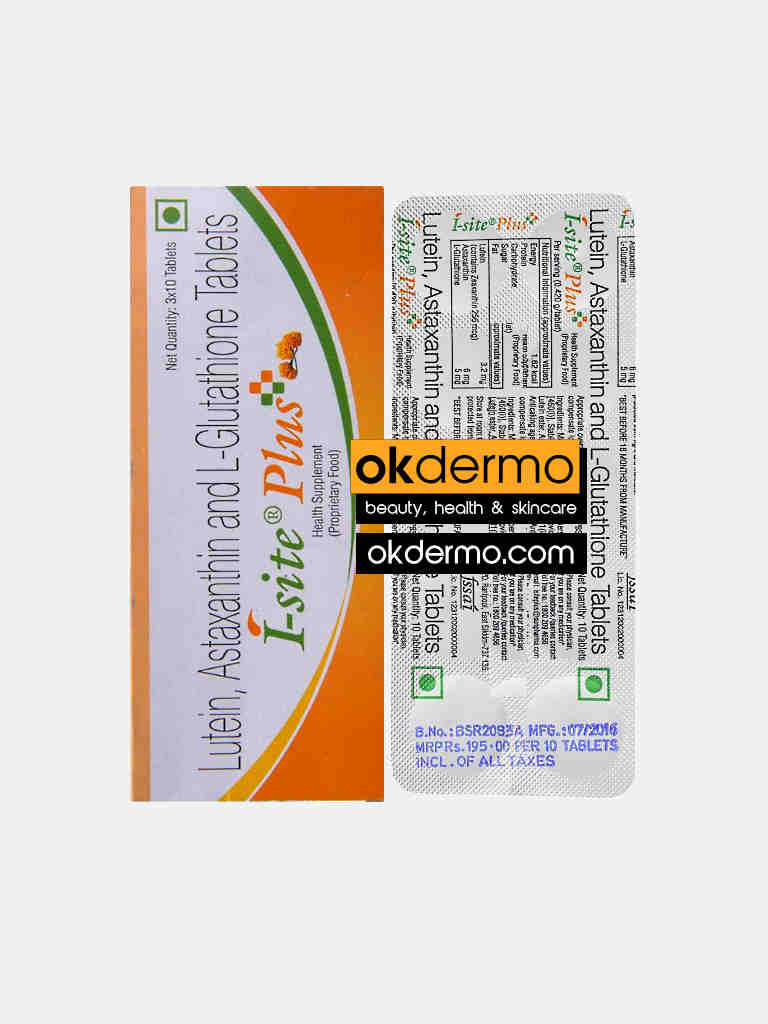
I-Site Plus® Eye Nutraceutical Tablets
Lutein 3.2mg + Astaxanthin 2mg + L-Glutathione 25mg
USD $28.00
I-Site Plus® Tablets is a health supplement used for various conditions related to eyes like age-related macular degeneration, cataracts, post-Lasik recovery, glaucoma, and retinitis pigmentosa. I-site Plus contains Lutein, Astaxanthin, and L-Glutathione as key ingredients that help in reducing damage in the center of the retina in people with age-related macular degeneration. Take one tablet twice a day or as directed by the physician.
- Effective in treating eye conditions like glaucoma and cataracts
- Helps prevent eye diseases
- Acts as a natural scavenger and protects eyes from the damage caused by free radicals
Size: 30 Tablets
| Title
| Range | Discount
|
|
Items: | 2 - 4 |
5%
USD $26.60
|
|
Items: | 5 - 8 |
10%
USD $25.20
|
|
Items: | 9 + |
15%
USD $23.80
|