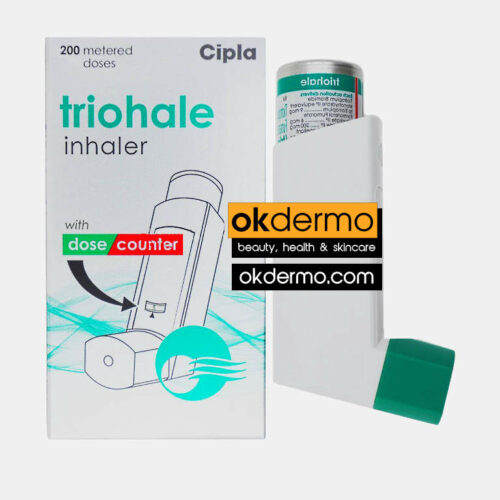
Description
What is Formoterol 6mcg + Tiotropium 9mcg Duova® Inhaler?
Duova® is an inhaler composed of two medications known as Tiotropium Bromide and Formoterol Fumarate. The cooperation of these two medications can relieve symptoms associated with chronic obstructive pulmonary disease (COPD).
COPD is a chronic condition that can not be cured with the help of medications. This condition arises with two other chronic lung conditions called chronic bronchitis and emphysema. As a result, the airways get narrowed, and the patients experience difficulties in gas exchange. The regular use of Duova inhalers can help these patients get rid of the symptoms. The best results can only be experienced when using the inhalers for about a week. Duova® inhalers belong to prescription-only medications where the physician determines the dosage depending on your age and condition. Duova® inhalers belong to maintenance medications, where this is not recommended in emergency conditions.
Tiotropium Bromide belongs to a drug class called an anticholinergic. Tiotropium Bromide get binds to the muscarinic receptors within the bronchial mucosal lining. Therefore they can inhibit the activation of cholinergic receptors. The activation of cholinergic receptors may result in Bronchoconstriction. The inhalation of anticholinergic drugs may help relax the airways, making it easy for the patients to breathe in and out.
Formoterol Fumarate is a type of bronchodilator that activates beta two receptors in the respiratory tree. Therefore Formoterol Fumarate is categorized under beta two agonists. Formoterol Fumarate can start the beta two receptors resulting in bronchodilation. COPD is a condition where the airway gets narrowed. The use of bronchodilators may help significantly in suppressing Bronchoconstriction. Therefore the normal bronchial lumen size can be obtained using these inhalers.
Side effects of Duova® Inhaler
Formoterol Fumarate and Tiotropium Bromide are from different drug classes and exhibit other actions within the body. Therefore they provide different side effects. There is a chance of initiating immediate hypersensitivity reactions. Hypersensitive reactions can occur when the consumer is allergic to the medications or any other excipient within the product. Therefore, close observation and allergy history of the patient is essential when prescribing the medicine for the first time.
Side effects of Tiotropium Bromide. The use of anticholinergic medications such as Tiotropium Bromide may result in dryness in the oral cavity, tachycardia, cough, palpitation, constipation, and urinary retention. These side effects may fade with time. If not, talk with the physician for more advice.
Side effects of Formoterol Fumarate. Heart muscles may contain beta receptors. Therefore Formoterol Fumarate causes side effects on the cardiovascular system. Shortly after the inhalation of Formoterol Fumarate, the consumer’s heart rate may increase. Therefore close monitoring is required at the initial stages until the body adapts to the medication.
Some patients may experience reduced serum potassium levels.
How to use Duova® Inhaler
Take the inhaler to the dominant hand and shake it gently for a few seconds. Remove the cover of the mouthpiece and exhale comfortably. Now seal the mouthpiece inside the mouth. Press the inhaler to release the medications. At the same time, start to breathe in and hold the breath for a few seconds. Now remove the inhaler from the mouth and breathe normally. Repeat the same procedure until the prescribed number of puffs is taken. Finally, wash the oral cavity with water. And replace the inhaler in a cool, dry place.
Precautions:
- Take precautions if you are allergic to Tiotropium Bromide and Formoterol Fumarate. Tell your allergy history to the physician.
- Avoid using Duova inhalers if you are pregnant or breastfeeding. Consult the physician for more details.
- If you experience any bronchospasms, avoid using the Duova inhalers.
- Avoid using Duova inhalers during labor as it can result in labor complications.
- Avoid Duova inhaler usage if you are below 18years.