Glaucoma Drops – Travoprost Ophthalmic Solution 0.004%
Table of Contents
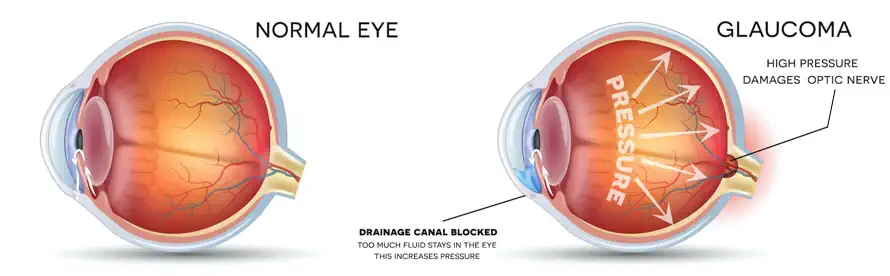
Travoprost Ophthalmic Solution 0.004% is used to treat glaucoma and ocular hypertension (a condition in which increased pressure in the eye causes vision loss). There are now several pharmacologic therapeutic medicines available for the treatment of glaucoma. The goal of treatment is to reduce IOP, which is a key risk factor in the course of the disease. Travoprost belongs to a family of drugs known as prostaglandin analogs. It reduces eye pressure by improving the flow of natural ocular fluids out of the eye. [1]
Benefits Of Travoprost Ophthalmic Solution 0.004%:
Several studies, such as the Advanced Glaucoma Intervention Study (AGIS), revealed an association between lowering IOP and retaining visual function. Topical IOP-lowering medicines may delay or prevent the development of primary open-angle glaucoma. The combination of travoprost and timolol resulted in greater IOP reductions than the positive control, timolol 0.5%, and reductions comparable to current travoprost + timolol. This study shows that a fixed combination of travoprost/timolol reduces IOP significantly and clinically in a once-daily dosing schedule. [4]
Travoprost 0.004% (40 g/mL; Travatan} has been found to reduce IOP and has been licensed for use in people who have primary open-angle glaucoma or ocular hypertension since 2001. Travoprost was authorized for use in children in Europe in 2014. Travoprost is successful and well-tolerated in a short retrospective study of children. After one month, 54% of pediatric glaucoma patients’ eyes responded to treatment, with an IOP drop of 15%. The most prevalent adverse effect was increased eyelash growth. Travoprost was given to pediatric patients as an adjuvant to existing glaucoma drugs in research, and 60% of patients responded, with a 10% drop in IOP. [2]
In Clinical Trials, people with open-angle glaucoma or ocular hypertension and baseline pressures of 25 to 27 mmHg who were given travoprost ophthalmic solution 0.004% once daily in the evening observed IOP reductions of 7 to 8 mmHg. In these studies’ subgroup analyses, the mean IOP drop in black patients was up to 1.8 mmHg larger than in non-black people. [3]
At this point, it is unknown if this distinction is due to race or highly pigmented irides. Patients with an average baseline IOP of 24 to 26 mmHg on timolol maleate ophthalmic solution 0.5% twice a day who had treatment with travoprost ophthalmic solution 0.004% dosed daily adjunctively to timolol maleate ophthalmic solution 0.5% twice daily showed 6 to 7 mmHg reductions in IOP in a multi-center, randomized, controlled trial. [3]
Dosing Of Travoprost Ophthalmic Solution 0.004%:
The Dosage of this medication will differ according to the patient. This information only includes the normal dosages of this drug. If your dose varies, do not change it until your doctor advises you to.
In addition, the number of dosages you take each day, the duration between doses, and the duration of time you take the drug are all determined by your health condition.
In the case of ophthalmic dose form (eye drops):
- For glaucoma or ocular hypertension:
Adults and children 16 and up—Use one drop in the affected eye once a day in the evening. It isn’t recommended for children under the age of 16.
How to use Travoprost Ophthalmic Solution 0.004%:
Before you begin using travoprost and each time you get a refill, read the patient guide. Apply this medicine to the affected eye(s), generally once a day in the evening, as directed by your doctor. Travoprost will not work as effectively if used more frequently.
Before using eye drops, cleanse your hands. To avoid contamination, avoid touching the dropper tip or allowing it to come into contact with your eye or anything else.
If your brand has the preservative benzalkonium chloride, remove your contact lenses before using this medication. Contact lenses can absorb this preservative. After using this medication, wait at least 15 minutes before putting your lenses back in.
What Side Effects May You Experience If You Take This Medication?
You should report the following side effects to your doctor or healthcare practitioner as soon as possible:
- Allergic responses such as skin rash, itching or hives, swelling of the cheeks, lips, or tongue
- Vision disturbances
- Swollen or infected eyes or eyelids
Side effects that do not normally necessitate medical treatment (but should be reported to your doctor or health care practitioner if they persist or are annoying):
- Immediate burning, stinging, or discomfort after applying the solution
- Changes in eye, lash, or eyelid color
- Dry eyes
Precautions:
Tell your doctor or pharmacist if you are allergic to travoprost, if you are allergic to similar medicines or if you have any other allergies. Inactive chemicals (such as preservatives like benzalkonium chloride present in some brands) in this product may cause allergic reactions or other complications. For more information, consult your doctor. Inform your doctor if you have other eye issues (such as macular edema, iritis, uveitis, lens extraction/aphakia).
Your vision may get temporarily blurred after using this medication. Do not drive, operate machinery, or engage in any activity that needs clear vision until you are confident that you can do so safely.
This drug should be taken only when needed during pregnancy. Consult your doctor about the risks and benefits.
- Store the medication at room temperature in a well-closed container away from heat, moisture, and sunlight. Prevent freezing. Keep out of children’s reach.
- Do not keep expired or no longer-needed medications. Consult with your doctor or pharmacist about how to properly dispose of any medications you no longer require.
References:
- (n.d.). Travoprost Ophthalmic. Retrieved from medlineplus.gov: https://medlineplus.gov/druginfo/meds/a602027.html
- (n.d) A 3-Month Safety And Efficacy Study Of Travoprost 0.004% Ophthalmic Solution Compared With Timolol In Pediatric Patients With Glaucoma Or Ocular Hypertension. Retrieved from nih.gov: https://www.sciencedirect.com/science/article/pii/S1091853116304438
- (n.d.). Travoprost Ophthalmic Solution, 0.004% Solution/ Drops. Retrieved from nih.gov: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=00dc284f-fd96-423b-bb8f-b90ee13439bf#:~:text=ocular%20hypertension%20…-,Travoprost%20ophthalmic%20solution%20(ionic%20buffered%20solution)%200.004%25%20is%20indicated,once%20daily%20in%20the%20eveni
- (n.d.). Efficacy And Safety Of A Fixed Combination Of Travoprost 0.004%/Timolol 0.5% Ophthalmic Solution Once Daily For Open-Angle Glaucoma Or Ocular Hypertension. Retrieved from nih.gov: https://www.sciencedirect.com/science/article/abs/pii/S0002939405002850
- ClevelandClinic. (n.d.). Travoprost Eye Solution. Retrieved from clevelandclinic.org: https://my.clevelandclinic.org/health/drugs/18714-travoprost-eye-solution
Post by:
Marcella Jiovanni
Skin Care Professional
“Marcella Jiovanni actively promotes the importance of maintaining healthy skin, she envisions the future of dermatology as moving away from pure medical, pharmacological dermatology and flowing more toward a holistic approach to wellness and skincare.”